
Two babies born in Scotland have become the first in Britain to be treated on the NHS with a potentially life-saving drug described as the world’s most expensive.
The one-off gene therapy is designed to prolong and transform the lives of children with spinal muscular atrophy, a rare condition.
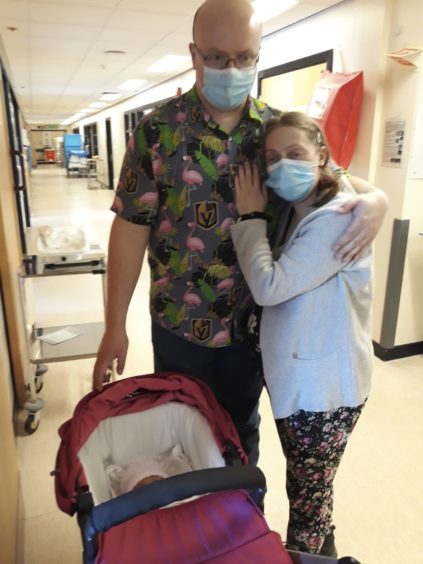
The Royal Hospital for Children in Glasgow – so far the only Scottish centre allowed to administer the drug, Zolgensma – delivered the UK’s first dose to a baby in February and now doctors have confirmed a second baby – Isabella Winfield from Morayshire – has received her life-changing dose of the drug, which has a list price of £1.79 million.
Six-week-old Isabella is the youngest child in the UK to have Zolgensma and one of the youngest in the world. The baby, from Archiestown, was given the treatment at 20 days old on May 20, just a week after diagnosis and five days before five-month-old Arthur Morgan, who was revealed last week as the first baby in England to receive the therapy.
Until recent years, there were no treatment options available for little ones with spinal muscular atrophy (SMA). Most babies born with the condition do not survive their first year. The emergence around four years ago of the drug Nusinersen helped prolong life, but Zolgensma – given in a one-off dose, ideally within the first six months of birth and potentially with no need for further treatment – is, according to specialists, a game changer with the potential to allow babies to sit, crawl and walk.
Isabella has the most severe form of the degenerative illness – Type 1 SMA – that weakens muscles and causes breathing difficulties. Doctors see only about three cases of SMA1 each year in Scotland.
Her parents IT trainer Richard Winfield, 36, and 35-year-old translator Margaret Paluszynska told The Sunday Post of their joy, disbelief and gratitude. Richard said: “We knew it would be a race against time to get the treatment to her. There are a number of things that need to be cleared before a child can qualify for Zolgensma. And without this treatment, or at least Nusinersen, babies with such a severe case of SMA don’t live long. We didn’t even want to think what she’d be going through without help”.
Margaret added: “I was thinking how the hell will we find the money to get Zolgensma to her in time before she deteriorates too far? I didn’t realise when doctors gave us the diagnosis that we would actually be able to get the treatment straight away. It was mind-blowing how prepared the medical teams were. It was thanks to them that it could be administered within such a short time. And I was even more amazed it was available in Scotland on the NHS, knowing what the costs are and how hard it is to obtain.”
Dr Iain Horrocks, the paediatric neurologist who leads the 10-strong team at the Glasgow children’s hospital, said: “We have treated two babies in Scotland so far, the first at the end of February this year. That baby’s parents are happy for me to say their child is doing really well.”
Horrocks said the younger babies are, the better they respond to the therapy. “Isabella glided through her treatment,” he said. “Because she was so young, she was the perfect candidate for it. We should see some real improvements with her in the next couple of months.
“It is amazing we can treat these children in Scotland, and long may it continue. There are 1,200 children in the world treated for this condition with this drug, but almost all of those are in drug trials.”
He added: “Zolgensma is a wonder drug. It is a true gene therapy. We replace the gene and no further treatment is potentially needed. Nusinersen, is a chemical, it is not the same.”
Zolgensma was made available on the NHS after the health service struck a deal with manufacturers Novartis Gene Therapies. The Scottish Medicines Consortium approved its use in the same month. It is the first gene therapy to be accepted by the SMC for use by the NHS in Scotland. England has four sites around the country from which Zolgensma can be administered. Horrocks said Northern Ireland’s first patient was treated in Belfast two weeks ago and the therapy has yet to be given in Wales.
Margaret – who is backing doctors’ calls for routine screening for SMA – has a family history of the condition but had not thought her child would be affected because there was no history of it on her partner’s side. She explained: “For SMA to manifest, it needs two carriers of the recessive gene or in extremely rare cases a mutation to occur in one parent.”
Their lives since Isabella’s birth on April 30 at Dr Gray’s Hospital in Elgin have been an “exhausting roller coaster”. Reliving the moment SMA was first suspected, she said: “The midwife was checking basic reflexes and one thing that stood out was a very low – and in the case of her left hand almost non-existent – muscle tone in her limbs and no tensing response to the drop-tests.” When the tests were repeated later, there was no improvement and the paediatric department was called in.”
After 12 days of further probes and monitoring to try to find the cause, she was transferred to Royal Aberdeen Children’s Hospital for further testing. At that time Isabella’s genetic blood test results came back positive for SMA1. “It was a possibility the medical staff must have already accounted for and Glasgow had been alerted to the potential case and was on standby to order a dose of Zolgensma as soon as possible,” her mum said.
Isabella was admitted to the Glasgow children’s hospital on May 19 and extensively screened to ensure she could be given the gene therapy safely. Margaret explained: “It is a treatment that can only be applied to a healthy, ideally pre-symptomatic baby, otherwise the risks become very severe.”
The treatment was given the following day. “The next morning we could already see small changes, like movements in her left hand,” said Margaret. “It was quite surprising. Even though doctors said it can make a difference within the first week, they were hardly anticipating to see anything significant so fast.”
After 48 hours, the family was allowed home but regular check-ups continue and Isabella is on a 30-day course of steroids to protect her from potential side-effects of the therapy.
Margaret admitted: “My first thought with the diagnosis was that if she doesn’t have a life-threatening condition she will never walk, or run, she will never do the things that any normal person wants to be doing.
“But the medical teams at all three hospitals did all they could to get her the best possible help as soon as humanly possible. So far, Isabella has been a star through all of this and is very well. Most of the time she is being what a baby should be, which is more than we had hoped for. We can see that she is getting livelier, getting bigger, and putting on weight. She might not run the marathon but we hope to see her walking and moving around like any child by the age of two.”
The drug
Spinal Muscular Atrophy Type 1 is the most severe form of the genetic condition and severely impacts on movement, muscle control and breathing. There is no cure for the condition caused by mutations in the SMN1 gene, but revolutionary treatments have emerged in the last few years.
The SMA condition emerges in the first six months of a baby’s life but more than two thirds of the children do not survive until their second birthdays.
Zolgensma, administered with an IV transfusion, contains a healthy copy of the missing or faulty gene SMN1. This is inserted into a harmless virus. In the body, the virus delivers the replacement gene into the nucleus of motor neuron cells. This is essential to prevent the cells from gradually dying. The now healthy motor neuron cells start producing the missing SMN protein which is vital for muscle function.
Now these children are not dying. Now we can give these children some hope
– Paediatric neurologist Iain Horrocks
The doctor leading the landmark treatment in Scotland has hailed the new drug as a game-changer.
Dr Iain Horrocks said: “The longer you leave it until you treat these babies, the more neurons they lose and the less you can recover by giving any treatments. Zolgensma is the pièce de résistance in treating this type of condition at the moment. The sooner we can treat them, as soon as they begin to decline, the better. Screening has to be the future.
“Baby Isabella was picked up at three days of age. The genetic test was done almost immediately and the result was back quickly. At two weeks of age, we had a diagnosis for her and we moved to treat her within three weeks.
“As a team we are excited about every baby who is treated, but we are really excited about her because she is so young, she is the gold standard candidate for this treatment.”
There already exists a group of children with SMA1 who are being treated with the drug Nusinersen, which emerged around four years ago. It is given in injections into the spine every four months.
Horrocks said: “Nusinersen is a chemical rather than true gene therapy. Zolgensma is a true gene therapy. We replace the gene, and no further treatment is needed potentially. Nusinersen is not the same. Those families whose children are treated with it are asking if they can get Zolgensma.”
However, he added: “Before Nusinersen, 90% of children with SMA died before one year of age. Now these children are not dying. Now we have this wonder drug Zolgensma – and Nusinersen – we can give them some hope.
“The expectation from Isabella is that she could go on to hit her motor milestones within the World Health Organisation classifications. We hope she will be able to have a life whereas a few years ago we would never have been able to talk about that.”
He said: “I am just shy of 50 and have been a consultant for 17 years. Having this turned on its head in the last few years re-enthuses you. I think I can do this for the next 15 or 20 years as it makes this all worthwhile.”
The support
Spinal Muscular Atrophy UK is a charity that has been working since 1985 to provide information and support to families affected by SMA. It aims to improve access to the best care, services and drug treatments today and funds research-related projects that can change tomorrow.
Support team manager, Liz Ryburn, said: “We’re delighted to see Scotland move so quickly to ensure this groundbreaking gene therapy reaches infants diagnosed with this devastating condition at the earliest possible opportunity. This is truly potentially life-changing. We now need newborn screening for SMA to be introduced at the earliest possible time.”
Find out more at smauk.org.uk

Enjoy the convenience of having The Sunday Post delivered as a digital ePaper straight to your smartphone, tablet or computer.
Subscribe for only £5.49 a month and enjoy all the benefits of the printed paper as a digital replica.
Subscribe
 © NEWSLINE MEDIA LIMITED
© NEWSLINE MEDIA LIMITED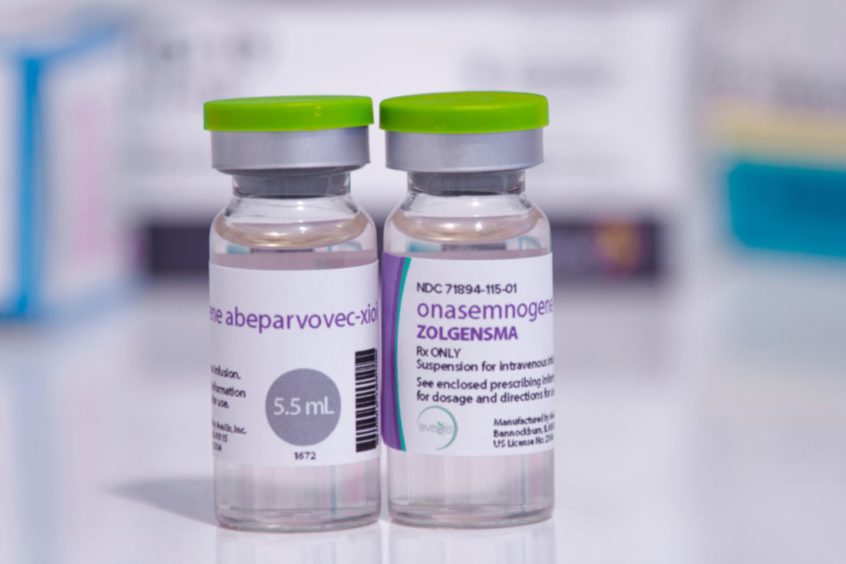 © Shutterstock
© Shutterstock